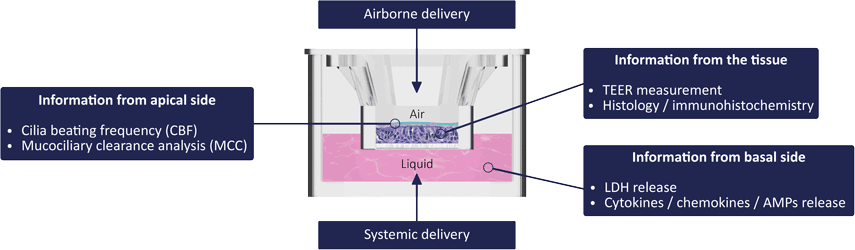
Scheme of repeated dose toxicity study for VGX-6150. C57BL/6 mice were... | Download Scientific Diagram
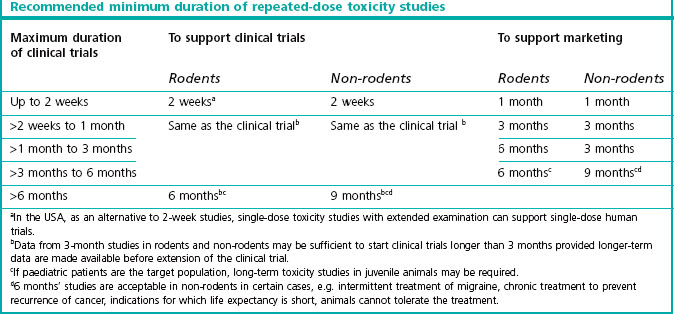
A No-Observed-Adverse-Effect Level of 1000 Mg/Kg in A 28-Day Repeated-Dose Study as a Limit Value for Acute Toxicity Testing

Appendix G: Summary of Acute- and Chronic-Toxicity Data in Various Mammalian Species (Ditran, Benactyzine, EA 2545, EA 3167, EA 3443, EA 3392, EA 3580, EA 3834, 226,086)-- by Leo G. Abood, Ph.D.

Foods | Free Full-Text | Single and Repeated Dose 28-Day and 13-Week Toxicity Studies of Oil Prepared from the Internal Organs of the Japanese Giant Scallop (Patinopecten yessoensis) in Mice
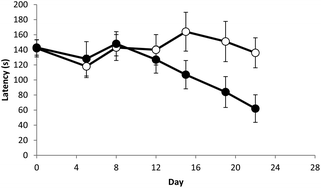
Functional assessments in repeat-dose toxicity studies: the art of the possible - Toxicology Research (RSC Publishing)

Molecules | Free Full-Text | Single, 14-Day, and 13-Week Repeated Dose Toxicity Studies of Daily Oral Gelidium elegans Extract Administration to Rats