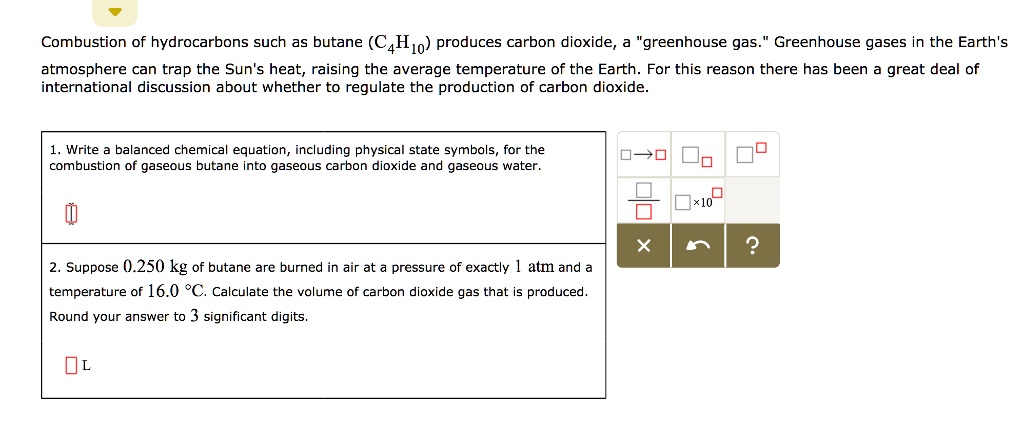
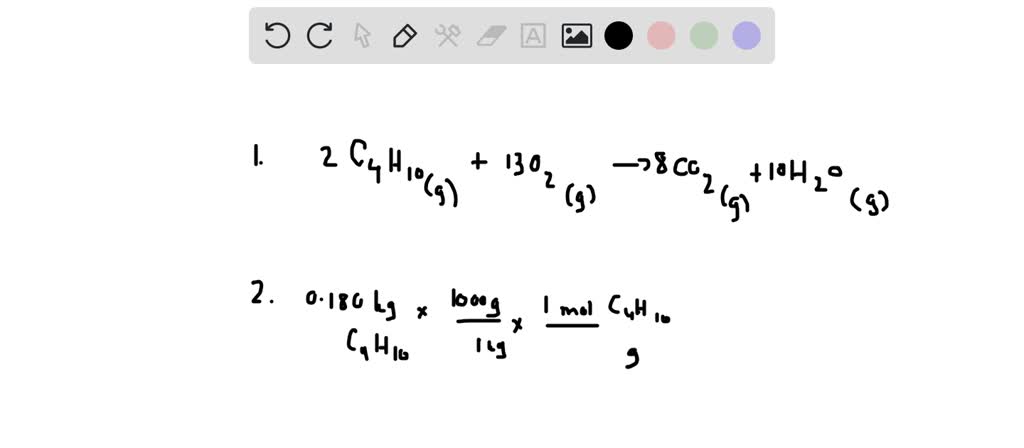
SOLVED: Combustion of hydrocarbons such as butane (C4H1o) produces carbon dioxide, greenhouse gas Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth; For
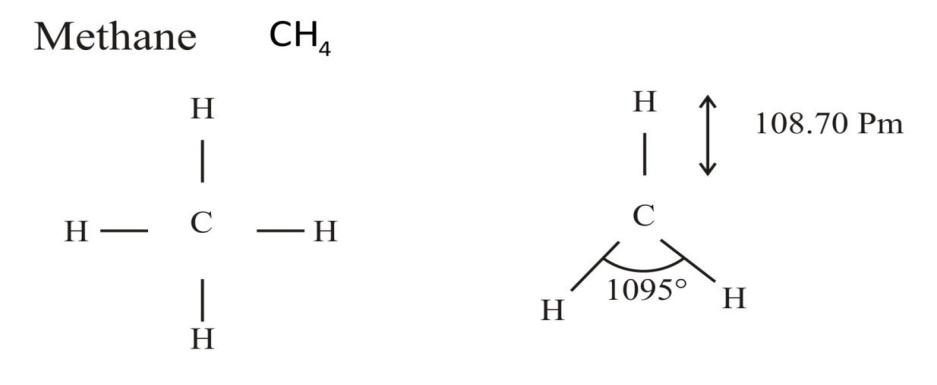
How will you convert methane to following (a) propane (b) butane (c) methyl butanoate (d) ethanoic acid (e) ethanol?

Blue Flame from Gas Hob Produce Greenhouse Gas Emissions. Kitchen Stove Grate on a Burner Fuelled by Combustible Natural Stock Image - Image of household, consumption: 236617471
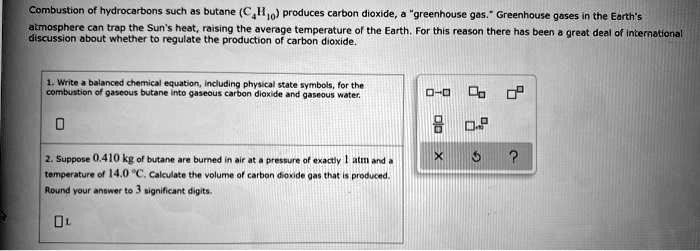
SOLVED: Combustion hydrocarbons such butane (€,Ila) praduces carbon dioxide "greenhouse gas. Greenhons goses In the Earth"Iimosphare can trap the Sun" heat, raising the averoge temperature the Earth For this reason thcre has

Assessment of Eco-friendly Gases for Electrical Insulation to Replace the Most Potent Industrial Greenhouse Gas SF6 | Environmental Science & Technology
Can propane or butane be produced using CO2 and H2 in a catalytic process - similar to how methane can be produced using the Sabatier reaction? - Quora

Which of the following is not a greenhouse gas? (A) Methane and nitrous oxide (B) Hydroflurocarbon (HFC) (C) Chloroflurocarbon (CFC) (D) Butane

Effect of ethane, propane, n-butane, iso-butane and nitrogen addition... | Download Scientific Diagram

Wholesale 100pcs Professional Industrial 10kw Lpg Propane Butane Indoor Portable Air Gas Heaters For Greenhouse Farm Workshop - Machine Centre - AliExpress
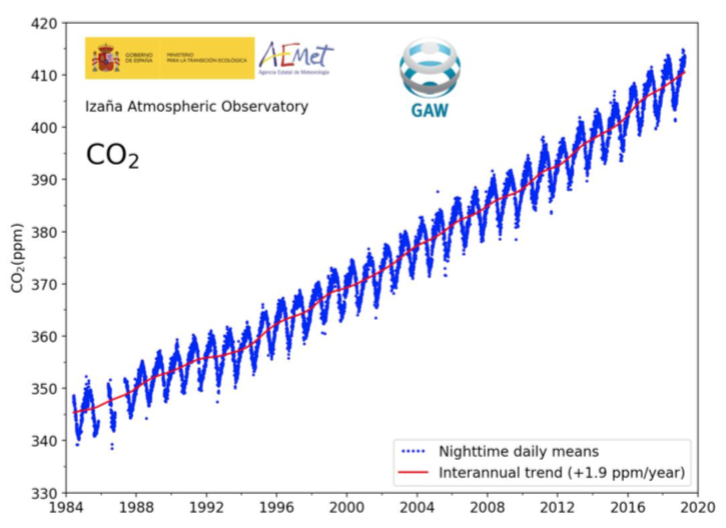
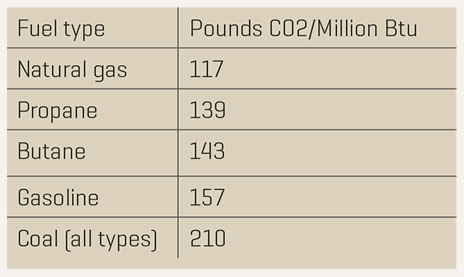


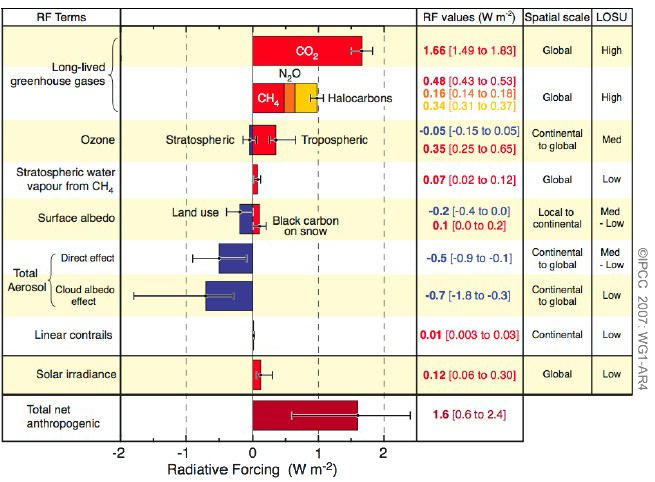
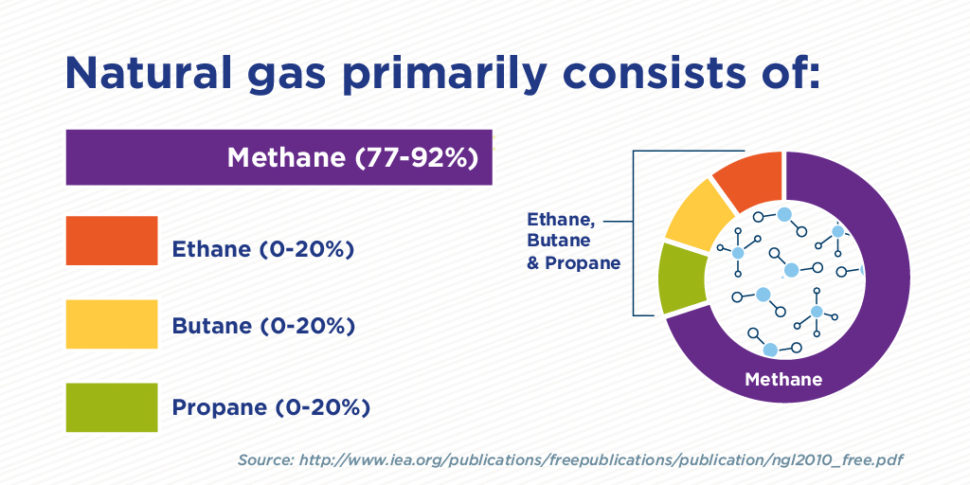



.png)


![PDF] Diffusion of Methane, Ethane, Propane, and n-Butane in Water from 25 to 43° | Semantic Scholar PDF] Diffusion of Methane, Ethane, Propane, and n-Butane in Water from 25 to 43° | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7a4a56203add29e0800d18448a8309e940197459/3-Table11-1.png)
